Introduction
Coronaviruses are enveloped viruses composed of a single-stranded, positive-sense RNA genome and belong to the family Coronaviridae.1 SARS-CoV-1 and SARS-CoV-2 are genetically similar severe acute respiratory syndrome coronaviruses and have been responsible for global epidemics. The SARS-CoV-2 is a novel coronavirus that emerged from Wuhan, China, and has been reported to cause COVID-19. Both viruses have similar genomes and mechanisms of transmission through direct contact or indirect contact, droplets, or any contaminated surfaces by an infected person.2 COVID-19 is a systemic disease involving multiple systems, including cardiac, gastrointestinal, respiratory, neurological, and hematological manifestations. The pathogenesis behind systemic manifestation is still unclear, but many studies have shown that it infects the human body by binding to the angiotensin-converting enzyme 2 (ACE2) receptors. ACE2 receptors are widely present in the heart, lungs, endothelium tissues of blood vessels, kidneys, and gastrointestinal tract and can potentially cause multi-system manifestations.3 These receptors are also present on platelets, and SARS-CoV-2 stimulates procoagulants which cause platelets aggregation and endothelial injury and release cytokines and other inflammatory markers.4 Hematological manifestations of COVID-19 include lymphopenia, eosinopenia, neutrophilia, thrombocytopenia, and, less frequently, thrombocytosis. Previous studies have shown that thrombocytosis is mainly due to cytokine storms with severe prognosis and longer hospitalization, but clear etiology remains unknown.5 We present the case of a 30-year-old African American female who developed extreme thrombocytosis due to COVID-19 and was treated with supportive management based on stable clinical status.
Case Report
A 30-year-old African American female patient presented to the emergency room with complaints of dyspnea and high-grade fever for three days. The patient denied sore throat, chest pain, abdominal pain, and diarrhea. Past medical history was significant for multiple sclerosis, well controlled with ocrelizumab with no episodes of remission. The patient had up-to-date immunization status along with the COVID-19 vaccine. On admission vitals, signs showed blood pressure 113/56 mmHg, heart rate 113 beats per minute, respiratory rate 28 breaths per minute, and spO2 95% on room air. The physical examination revealed bilateral crackles on chest auscultation, and other systemic examinations were unremarkable. Initial laboratory workup showed an elevated white blood cell count (WBC) of 18.5 × 103/mcL and a normal platelet count of 264 × 103/mcL in the emergency department. Chest x-ray showed bilateral multifocal consolidations. The patient tested negative for COVID-19 polymerase chain reaction (PCR) and for the rapid influenza antigen test. She was treated with broad-spectrum antibiotics for pneumonia. Blood cultures ½ showed growth of Haemophilus influenzae biotype 2. After 48 hours, we discontinued intravenous antibiotics and started the patient on oral antibiotics based on her down-trending WBC count and lack of supplemental oxygen requirement. During hospitalization, on day three, CBC showed a platelet count of 540 × 103/mcL, which we believe was due to sepsis response. On day six of hospitalization, the patient complained of chest pain, shortness of breath, sore throat, low-grade fever, and watery diarrhea.
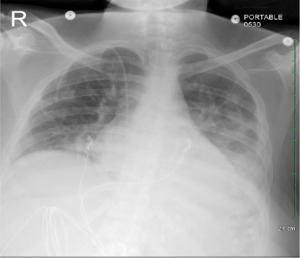
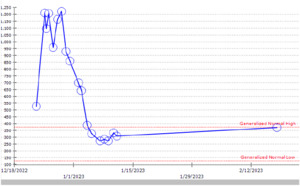
Computed tomography angiography (CTA) chest to rule out pulmonary embolism was negative, and the chest x-ray showed new bilateral opacities (Figure 1). A complete blood count revealed a normal white blood cell count and an isolated jump in platelet count to 1209 × 103 /mcL from 264 × 103 /mcL (Figure 2). The patient tested positive for COVID-19 on a nasopharyngeal swab PCR test. The initial COVID-PCR test was negative, and we believe the patient had a hospital-acquired infection. We started supplemental oxygen, as the patient maintained SpO2 88% on room air, dexamethasone, and remdesivir, and consulted hematology/oncology for isolated extreme thrombocytosis. An extensive workup was conducted to rule out myeloproliferative disorder, including Janus kinase 2 (JAK2) mutation, BCR/ABL fusion, iron deficiency anemia, and acute phase reactants, including C-reactive protein, erythrocyte sedimentation rate, and interleukin6 and 8 (IL-6, IL-8). The patient was discharged after clinical improvement in symptoms, with outpatient follow-up for a myeloproliferative disorder workup, which turned out to be negative.
The patient was on ocrelizumab for MS, and no change in platelet count was noticed before and after starting the medication during previous visits; Naranjo’s assessment score was 0; we believe this isolated extreme thrombocytosis was due to COVID-19. Per our knowledge and literature review, extreme thrombocytosis due to coronavirus disease has rarely been reported in the literature.6
Discussion
We reported a case of extreme thrombocytosis in a previously vaccinated patient due to COVID-19. The patient had a normal platelet count, which jumped to 1.2 million during hospitalization. Based on the previous data, a much smaller proportion of COVID-19 patients present with thrombocytosis (5%) as compared to thrombocytopenia (19%).7 Thrombocytopenia is a common presentation in COVID-19; the mechanism behind it is platelet destruction, decreased platelet production, and increased removal of platelets. Thrombocytopenia can also occur due to coagulation activation, endothelial injury in blood vessels, and trapping of megakaryocytes in lung tissue injury. Thrombocytopenia is a prognostic factor for COVID-19.8 We have reviewed the literature, and there is minimal data regarding thrombocytosis due to COVID-19, while only one case of extreme thrombocytosis is reported.6 The pathogenesis behind SARS-CoV-2 transmission in the host is through ACE2 receptors on platelets, which cause the breakdown of spike proteins, and SARS-CoV-2 adheres to the cell membrane of the host cells. Spike proteins are one of the structural proteins of SARS-CoV-2, which can directly stimulate platelet activation.9 The disease course for SARS-CoV-2 includes three phases, the initial phase caused by active infection, the secondary pulmonary phase, and the hyperinflammatory phase caused by cytokine storm.6 Some previous studies have revealed that thrombocytosis is due to the third hyperinflammatory phase. The pro-inflammatory cytokines are interleukin-1B (IL-1B), interleukin-6 (IL-6), interleukin-12 (IL-12), interferon-γ (IFNγ), IFNγ-inducible protein 10 (IP10), and monocyte chemoattractant protein 1 (MCP1), which are associated with pulmonary inflammation and extensive lung damage.10 These inflammatory markers cause platelet activation, aggregation, and endothelial damage to blood vessels. Various hematological manifestations related to COVID-19 include lymphopenia, neutrophilia, eosinopenia, thrombocytopenia, thrombocytosis, and, rarely, extreme thrombocytosis. Thrombocytosis is defined as a platelet count of ≥ 450 × 109 /L, while a platelet count of >1,000 × 109 /L is defined as extreme thrombocytosis as per literature.6 The most common causes of thrombocytosis are acute infection, malignancies, myeloproliferative disorders, steroids, chronic inflammatory disorders, and hereditary thrombocytosis.11 There is limited data available regarding COVID-19-associated thrombocytosis. A study of a meta-analysis of 157 patients with the coronavirus-associated severe acute respiratory syndrome (SARS) revealed thrombocytopenia in (55%) of the patients during the initial course, which turned into reactive thrombocytosis in (49%) of the patient during the third week of the hospitalization. No evidence of thromboembolism was found.12 Thrombopoietin (TPO), von Willebrand factor (VWF), and other inflammatory markers, including I-6 and interleukin-3 (IL-3), stimulate megakaryocytes in the bone marrow and increase platelet production. COVID-19 causes cytokines release, which leads to increased platelet production. TPO plays a major role in megakaryocyte stimulation in lung tissue.11,12 Thrombocytopenia is a major severity prognostic factor for COVID-19; however, there is no data for the prognostic significance of extreme thrombocytosis in patients with COVID-19. There is a cohort study by Marko Lucijenic et al. Of 5879 hospitalized patients with COVID-19 with normal baseline platelet counts were monitored in the hospital course. During hospitalization (19.1%) of patients developed thrombocytopenia, and (4.6%) had thrombocytosis, with no case of extreme thrombocytosis. Based on the study results, elevated platelets were associated with a longer duration of symptoms, increased myelopoiesis, and a more mature inflammatory profile consisting of elevated WBCs, CRP, and D-dimers, and lower ferritin and IL-6. Patients with thrombocytosis also had overall better survival than patients with thrombocytopenia; however, patients with thrombocytosis had higher chances of developing thromboembolism than patients with normal and low platelet counts.6,8,11 Our patient required minimal supplemental oxygen and was treated with dexamethasone, remdesivir, fluids, and prophylaxis for deep venous thrombosis. We had not started low-dose aspirin or hydroxyurea as we ruled out myeloproliferative disorders and malignancies. The platelet count started trending down slowly after 48 hours, along with improved clinical status. The patient was followed on an outpatient basis and was found to have a baseline platelet count with no complications. COVID-19 patients can present with extreme thrombocytosis, but extensive workup should be done to rule out infectious, autoimmune, genetic mutations, malignancies, and myeloproliferative disorders. Close blood count monitoring is recommended for the worsening of the condition and progression of disease in COVID-19 patients. Limited data are available regarding the exact etiology and prognosis of the disease in patients with extreme thrombocytosis.
Further studies are necessary for the exact mechanism of this hematological manifestation.
Conclusion
Thrombocytopenia is a common hematological manifestation of COVID-19; however, in rare cases, patients can present with extreme thrombocytosis. COVID-19-induced thrombocytosis is associated with a better prognosis but a higher risk of venous thromboembolism. Clinicians should closely monitor platelet count during hospitalization and on outpatient follow-up. There should be extensive workup to rule out myeloproliferative disorders and hereditary thrombocytosis.